|
Republished
by permission from: http://www.ul.ie/~childsp/CinA/Issue43/editorial43.html
Article
originally published in 1994. Downloaded on 12, Oct 2003
The life and legacy of Antoine-Laurent Lavoisier
A chemical revolutionary May 1794 26th.
August 1743- 8th.
Peter E.Childs
Introduction
It is 200 years this year since the greatest chemist of all time
- Antoine-Laurent Lavoisier - lost his head to the sharp edge of the guillotine. Coffinhal, president of the tribunal that
condemned Lavoisier to death, said in mockery: "The Republic has no need of scientists" but Lagrange, the great French mathematician,
voiced the true assessment of that fateful May day 200 years ago:
"It only took a moment to cut off that head, and 100 years
perhaps will be required to produce another like it." (Il ne leur a fallu qu'un moment pour faire tomber cette tte, et cent
annes peut-tre ne suffiront pas pour en reproduire une semblable.)

Time has only underscored Lagrange's assessment of Lavoisier.
He died at the age of 51 at the height of his creative powers and after establishing modern chemistry on a firm footing. Who
knows what more he might have achieved had he lived? Every modern chemist, indeed every chemist for the last 200 years, lives
in the shadow and on the legacy of Lavoisier. E.J. Holmyard in his book (Chemistry to the time of Dalton (OUP, Oxford
1925) identified Lavoisier and Dalton as the founders of modern chemistry:
"The coping-stones of eighteenth century chemistry, which
are at the same time the foundation-stones of the modern science, were laid by Antoine Laurent Lavoisier (1743-1794) and John
Dalton (1766-1844)."
In 1994 we celebrate the 200th. anniversary of the death of Lavoisier
and the 150th. anniversary of the death of Dalton, and coincidentally the death of Linus Pauling, the greatest chemist of
the 20th. century.
It is instructive 200 years later to look back at Lavoisier's
life and achievements, to the very birth of modern chemistry. Before he died the phlogiston theory was dead, although some
chemists like Joseph Priestley refused to acknowledge it in their lifetimes, and the oxygen theory of combustion and respiration
was firmly established. Lavoisier laid his personal claim to be the author of the chemical revolution in 1792:
"This theory is not, as I have heard it called, the theory
of the French chemists in general, it is mine, and it is a possession to which I lay claim before my contemporaries
and before posterity. Others, no doubt, have given it new degrees of perfection, but I hope that one will not be able to deny
me the whole theory of oxidation and combustion; the analysis and decomposition of air by metals and combustible bodies; the
theory of the formation of acids; more exact knowledge of a great number of acids, notably vegetable acids;the first ideas
on the composition of plant and animal substances, and the theory of respiration."
A chronology of the main events in Lavoisier's life is given
in Box 1.
His early years
Antoine-Laurent Lavoisier was born on August 26th. 1743 in the
Marais district of Paris, the first child of Jean-Antoine Lavoisier (a lawyer) and Emilie (ne Punctis), the daughter of a
well-connected lawyer. A sister, Marie, was born in 1745 and in 1748 his mother died. They all went to live with Mme Punctis,
his maternal grandmother. Lavoisier stayed in this house until he was married in 1771. From 1754 to 1760 he went to school
at the College-Mazurin, where he won several prizes, including one in his second year for industry in his studies! In 1760
his sister died at the age of 15.
On leaving school he entered the School of Law and qualified
as a Bachelor of Law in 1763 and Licentiate in 1764. At school his initial interest was in art and literature and he wanted
to be a writer. Later he developed an interest in science while still at school and while studying law he developed this interest
further in his spare time under a number of remarkable teachers: the astronomer and mathematician de Lacaille, the botanist
de Jussieu, the geologist Guettard and the chemist Rouelle. Lavoisier's science was marked by its breadth of interest and
this background in a number of scientific disciplines, as well as his classical education, was a vital part of his later success
in many fields of science. As well as attending their lectures he also worked with them in the laboratory and did fieldwork,
learning practical skills as well as theory.
_________________________________
Box 1 Chronology of Lavoisier's life
26/8/1743 Born in Paris
1754-1760 Attended the Collége Mazurin in Paris
1763 Bachelor of Law
1763 Attended chemistry lectures by G.F.Rouelle
1765 Gold medal of the Academy of Sciences for a paper on lighting
a city
1768 Elected member of the Academy of Sciences
1768 Joined the company
Fermé-Génerale, which collected taxes for the government
1771 Married Marie Paulze (then aged 14)
1772 Started to investigate combustion and the calcination of
metals
1774 Published Opuscules Physiques et Chymiques
1774 Joseph Priestley visits in Paris in October
1775 Reports on the production and properties of vital air
1775 Joined the new gunpowder commission, Regie des Poudres
1776 Moved to live and work at the Royal Arsenal (until 1792)
1778 Started scientific farming at Fréchines
1783 Sur la Chaleur (with Pierre Laplace)
1783 Demonstrated the formation and decomposition of water
1787 Methode de
Nomenclature Chimique (with de Morveau, Berthollet and Fourcroy)
1789 Published Traité
Élémentaire de Chimie (The Elements of Chemistry)
1789-90 Studied human metabolism (with A.Séguin)
1791 The Fermé-Génerale suppressed
1792 Started work
on a complete edition of his memoirs (intended to be in eight volumes)
1792 Resigned his
post with the Gunpowder Commission and left the Royal Arsenal
4/11/1793 Arrest of former members of the Fermé-Génerale ordered
28/11/1793 Lavoisier gives himself up and is imprisoned
7-8/5/94 Lavoisier
tried before the Revolutionary Tribunal and condemned on false charges
8/5/1794 Lavoisier
guillotined with his fellow farmer-generals and his father-in-law
___________________________________
He studied chemistry under Guillame-Francois Rouelle who taught
chemistry at the Jardin du Roi from 1742-1768. Rouelle had an international reputation as a teacher and is remembered for
his classification of salts by their crystal forms, and for his chemical lectures. He was a great popularised of chemistry
and like Davy and Faraday at a later date in England, society flocked to hear Rouelle's demonstration lectures. He was the
demonstrator in chemistry and it was the custom in France at that time for the Professor of Chemistry and his demonstrator
to give the lectures in tandem: the Professor would first deal with the chemical theory and leave, and then the demonstrator
would take over and follow with experiments to support the Professor's ideas. Rouelle's Professor was Boudelin and he would
end his part of the lecture by saying:
"Such, gentlemen, are the principles and the theory of this
operation, as the demonstrator will now prove to you by his experiments."
He would then leave and Rouelle would enter. Unfortunately, Rouelle's
experiments often disproved or contradicted the Professor's theory, but people flocked to see Rouelle's demonstrations. He
was the greatest chemical educator of his time and Lavoisier was to be his most famous pupil. Rouelle came in full dress and
as the lecture proceeded he would strip off item by item, hanging has clothes on bits of apparatus, as he warmed up. Lavoisier
obtained a copy of Diderot's notes on Rouelle's lectures, which he studied at his leisure, adding his own notes and comments.
He also worked with Rouelle in his laboratory.
As well as chemistry, Lavoisier extended his studies to anatomy,
mathematics and meteorology, which was to become one of his life-long interests (much like John Dalton) and he made regular
observations for 30 years.
His scientific work
Lavoisier's scientific career was to last only 30 years but he
packed an immense amount of work into that period, despite many other activities that took up his time. He published his first
chemical memoir (or paper) when he was only 22 and in 1768 he was elected as a member of the prestigious Académie des Sciences,
France's most important scientific organisation (equivalent to the Royal Society in England). Around the same time he made
the fateful move of seeking to increase his personal wealth (based on legacies) by becoming a member of the Fermiers-Généraux
(Farmers-General), a tax-collecting syndicate. His membership of this reviled institution, despite his own irreproachable
conduct and honesty, was to be the direct cause of his arraignment by the forces of the French Revolution and his execution
a quarter of a century later.
From 1770 to 1775 he performed his key experiments on the combustion
of first non-metals and then metals which were to destroy both the phlogiston theory and also Boyle's idea that heat, a material
substance, passed through the container from the fire and resulted in an increase in mass when a metal was heated in air.
Lavoisier disagreed with Boyle, as he said:
"If the increase in weight of metals calcined in closed vessels
is due, as Boyle thought, to the addition of the matter of flame and fire which penetrates the pores of the glass and combines
with the metal, it follows that if, after having introduced a known quantity of metal into a glass vessel, and having sealed
it hermetically, one determines its weight exactly; and that if one than proceeds to the calcination in a charcoal fire, as
Boyle did; and lastly that if one then reweighs the same vessel after the calcination, before opening it, its weight ought
to be found to have increased by the whole of the quantity of the matter of fire which entered during the calcination.
If, on the contrary ... the increase in weight of the metallic
calx is not due to the combination of the matter of fire nor to any exterior matter whatever, but to the fixation of a portion
of the air contained in the space of the vessel, the vessel ought not to weigh more after the calcination than before, it
ought merely to be found partly empty of air, and the increase in weight of the vessel should take place only at the moment
when the missing portion of air is allowed to enter."
Lavoisier put his ideas to the test by careful, systematic experiments
using the most sensitive balance he could obtain. He had this to say on the importance of careful measurement:
"As the usefulness and accuracy of chemistry depend entirely
upon the determination of the weights of the ingredients and products, too much precision cannot be employed in this part
of the subject, and for this purpose we must be provided with good instruments."
The results clearly showed that his ideas were correct and the
increase in weight of a metal when heated to form a calx (oxide) was due to combination with part of the air, not to the absorption
of the hypothetical 'matter of fire', nor to the equally hypothetical phlogiston. Other experiments were done to consolidate
his views and he then could state categorically in 1774:
First, that one cannot calcine an unlimited quantity of tin
in a given quantity of air;
Second, that the quantity of metal calcined is greater in
a large vessel than in a small one, although it cannot yet be affirmed that the quantity of metal calcined is exactly proportional
to the capacity of the vessels.
Third, that the hermetically sealed vessels, weighed before
and after the calcination of the portion of tin they contain, show no difference in weight, which clearly proves that the
increase in weight of the metal comes neither from the material of the fire nor from any matter exterior to the vessel.
Fourth, that in every calcination of tin, the increase in
weight of the metal is, fairly exactly, equal to the weight of the quantity of air absorbed, which proves that the portion
of the air that combines with the metal during the calcination, has a specific gravity nearly equal to that of atmospheric
air.
I may add that, from certain considerations drawn from actual
experiments made upon the calcination of metals in closed vessels, considerations which it would be difficult for me to explain
to the reader without going into too great detail, I am led to believe that the portion of the air which combines with metals
is slightly heavier than atmospheric air, and that that which remains after the calcination is, on the contrary, rather lighter.
Atmospheric air, on this assumption, would form, relatively to the specific gravity, a mean result between these two airs."
At this point (October 1774) an important event occurred when
Lavoisier met the English chemist, Joseph Priestley, who was visiting France. Priestley told Lavoisier of his recent discovery
of dephlogisticated air (oxygen) by heating mercury calx (oxide). Lavoisier immediately linked this new gas with his active
component of the atmosphere and over the winter of 1774-75 he repeated and extended Priestley's experiments. He heated the
red calx of mercury with carbon and obtained mercury and 'fixed air' (carbon dioxide); he then heated the red calx alone and
obtained 'respirable or vital air'. He investigated these changes quantitatively and also studied the chemical and physiological
properties of the new gas, repeating Priestley's work. He reported his findings to the Acadmie des Sciences in 1775 (without
acknowledging Priestley's work) but his conclusions he drew from his measurements were his own:
"It thus appears to be proved that the principle which combines
with metals during their calcination, and which increase their weight, is nothing else than the purest portion of the very
air which surrounds us, which we breathe, and which passes, during this operation, from the gaseous state to the solid state;
if, therefore, one obtains it in the from of fixed air in all metallic reductions where carbon is used, this effect is due
to the combination of the carbon with the pure portion of the air. It is, indeed, very probable that all metallic cases would,
like that of mercury, give nothing but 'eminently respirable air' if one could reduce them all without the addition of any
other substances, as one reduces red precipitate of mercury per se."
(His failure to acknowledge what he borrowed from Priestley in
his work on oxygen, and later in 1783 what he borrowed from Cavendish in his work on the composition of water, have generated
immense controversies between the English and French schools as to who discovered what first. This is well illustrated by
the 'discussion' by T.E.Thorpe in Essays on Historical Chemistry chs. VI and VII, Macmillan, London 1902)
His crucial experiment is the one which has come to be called
"Lavoisier's Experiment", and is described in Box 2 in his own words, taken from the 1789 Traité Élémentaire de la Chimie
(Translated into English in 1790 as The Elements of Chemistry, a great title for a great book and still available as
a Dover reprint). Notice the clarity of the language and style in which it is written (in the first person), and the emphasis
on describing exactly what was done and on measuring the changes observed quantitatively. It is not cluttered with theory
or strange terminology, and it says no more and no less than the experiment demonstrates.
He had not yet publicly attacked the phlogiston theory, although
he made no use of it in his papers and he had been steadily demolishing its foundations with his experiments. However, in
1783 he published Reflexions sur Phlogistique (Reflections on Phlogiston), which made his position clear and
also brought out the opposition. He destroyed the citadel of Phlogiston with his careful experiments, his rigorous logic and
his well-chosen words:
"It is time to recall chemistry to a more rigorous method
of reasoning; to strip away the facts with which this science is enriched every day from that which reasoning and prejudices
add thereto; to distinguish fact and observation from that which is systematic and hypothetical; finally, to mark the limit,
so to speak, to which chemical knowledge has arrived, in order that those who follow us may set out with confidence from this
point to advance the science ..
Chemists have made of phlogiston a vague principle which is
not rigorously defined, and which consequently adapts itself to all explanations into which it may be brought. Sometimes this
principle is heavy, and sometimes it is not; sometimes it is free fire, sometimes it is fire combined with the earthy element;
sometimes it passes through the pores of vessels, and sometimes they are impenetrable to it. It explains at once causticity
and non-causticity, transparency and opacity, colours and the absence of colours. It is a virtual Proteus which changes its
form at every instant."
Since 1775 he had done many more experiments and showed that
non-metals combined with his respirable air to give acids. However, he now decided to change his name for this 'vital air'
that Priestley had discovered:
"I shall for the future call dephlogisticated air or eminently
respirable air by the name of the acidifying principle, or, if the same meaning is preferred in a Greek word, by that of the
oxygine principle."
In English this new name became oxygen. Professor Henry Armstrong,
the famous 19th. century English chemist and educator of, said this of Lavoisier's naming of oxygen:
"In designing the word Oxygen Lavoisier rose to the greatest
height of his unparalleled genius. Not only is the word a monument to his astounding insight into chemical phenomena, to his
philosophic power; it is also proof of a deep philological feeling and acumen, as well as of his sense of the beauty of words.
Think of the astounding step he took, after his instant appreciation of Priestley's discovery, in translating the old nonconformist's
ponderous reminder of the doubtful past of our science conveyed in the name Dephlogisticated Air into an all-significant
word of the aural and lingual perfection of Oxygen .... think of him as the pioneer who not only sought to put system into
the souls of chemists but also tipped their tongues with harmony."
Lavoisier had overturned over 50 years of chemical theory and
had stepped on the toes of all the famous and influential chemists of his time. Many older chemists rejected his views and
stuck to the old theory. The Swedish chemist Scheele wrote to Bergman in 1784:
"Would it be so difficult to convince Lavoisier that his system
of acids is not to everybody's taste? Nitric acid composed of pure air and nitrous air, aerial acid of carbon and pure air,
sulphuric acid of sulphur and pure air!.... Is it credible? ... I will rather place my faith in what the English say."
The Irish chemist Richard Kirwan initially rejected the new ideas
but then in 1791 publicly accepted Lavoisier's views:
"At last I am laying down my arms and abandoning phlogiston.
I see clearly that there is no authentic experiment in which the production of fixed air from pure inflammable air has been
demonstrated, and that being so, it is impossible to maintain the system of phlogiston .. I myself will give a refutation
of my own essay on phlogiston."
Henry Cavendish and Joseph Priestley remained believers in phlogiston
until their deaths, although Black in Edinburgh, Klaproth in Germany, Bergman in Sweden, and most American and Russian chemists
accepted the validity of Lavoisier's views. The chemical revolution, a relatively bloodless coup, was over during Lavoisier's
short lifetime, although in his paper of 1785 he himself was more pessimistic of the adoption of his new ideas:
"I do not expect that my ideas will be adopted all at once;
the human mind adjusts itself to a certain point of view, and those who have looked at nature from one standpoint, during
a portion of their life, adopt new ideas only with difficulty; it is, then, for time to confirm or to reject the opinions
which I have brought forward. Meanwhile, I see with great satisfaction that young people who begin to staidly the science
without prejudice, that mathematicians and physicists who come fresh to chemical truths, no longer believe in phlogiston in
the way in which Stahl presented it, and look upon the whole of this doctrine as a scaffolding more embarrassing than useful
for the continuance of the structure of chemical science."
In 1791 Joseph Black wrote to Lavoisier, as follows:
"The numerous experiments which you have made on a large scale,
and which you have so well devised, have been pursued with so much care and with such scrupulous attention to details, that
nothing can be more satisfactory than the proofs you have obtained. The system which you have based on the facts is so intimately
connected with them, is so simple and so intelligible, that it must become more and more generally approved and adopted by
a great number of chemists who have long been accustomed to the old system .... Having for thirty years believed and taught
the doctrine of phlogiston as it was understood before the discovery of your system, I, for a long time, felt inimical to
the new system, which represented as absurd that which I had hitherto regarded as sound doctrine; but this enmity, which springs
only from the force of habit, has gradually diminished, subdued by the clearness of your proofs and the soundness of your plan."
Another important contribution Lavoisier made to chemistry was
to revise the names used in chemistry. He worked on this for several years (1782-1787) with de Morveau, Berthollet and Fourcroy,
and in 1787 they published Methode de Nomenclature Chimique (Methods of Chemical Nomenclature). If Lavoisier had done
nothing else this alone would have ensured his place in chemistry's Hall of Fame. Until this time the language of chemistry
was a confusing mess of unsystematic names, with many different names for the same substance and no way of relating the name
to the chemical composition. Overnight Lavoisier and his French colleagues swept away the alchemical accretions of centuries
and pot in place a system, which remained essentially unchanged until this century and still provides the basis of chemical
nomenclature. Chemistry was full of compounds such as Epsom Salts, Fuming Liquor of Libavius, Butter of Arsenic, Oil of Vitriol
etc. Lavoisier had the job of drawing up and explaining the principles on which the new nomenclature was to be founded.
Box 2 Lavoisier's Experiment
"I took a matrass [A] of about 36 cubical inches capacity,
having a long neck BCDE, of six or seven lines internal diameter, and having bent the neck as in [the figure], to allow of
its being placed in the furnace MMNN, in such manner that the extremity of its neck E might be inserted under the bell-glass
FG, placed in a trough of quicksilver RRSS; I introduced four ounces of pure mercury into the matrass, and, by means of a
syphon, exhausted the air in the receiver FG, so as to raise the quicksilver to LL, and I carefully marked the height at which
it stood, by pasting on a slip of paper. Having accurately noted the height of the thermometer and barometer, I lighted a
fire in the furnace MMNN, which I kept up almost continuously during twelve days, so as to keep the quicksilver always very
near its boiling point.
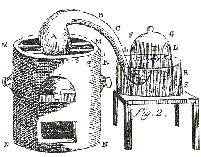
|
| Figure 1. Lavoisier's Apparatus. |
Nothing remarkable took place during the first day: the mercury,
though not boiling, was continually evaporating, and covered the interior surface of the vessel with small drops, at first
very minute, which gradually augmenting to a sufficient size, fell back into the mass at the bottom of the vessel. On the
second day, small red particles began to appear on the surface of the mercury; these, during the four or five following days,
gradually increased in size and number, after which they ceased to increase in either respect. At the end of twelve days,
seeing that the calcination of the mercury did not at all increase, I extinguished the fire, and allowed the vessels to cool.
The bulk of the air in the body and neck of the matrass, and i the bell-glass, reduced to a medium of 28 inches of the barometer
and 54.5o of the thermometer, at the commencement of the experiment was 50 cubical inches. At the end of the experiment
the remaining air, reduced to the same medium pressure and temperature, was only between 42 and 43 cubical inches; consequently
it had lost about 1/6 of its bulk. Afterwards, having collected all the red particles, formed during the experiment, from
the running mercury in which they floated, I found these to amount to 45 grains.
I was obliged to repeat this experiment several times, as
it is difficult in one experiment both to preserve the whole air upon which we operate, and to collect the whole of the red
particles, or calx of mercury, which is formed during the calcination. It will often happen in the sequel, that I shall, in
this manner, give in one detail the results of two or three experiments of the same nature.
The air which remained after the calcination of the mercury
in this experiment, and which was reduced to 5/6 of its former bulk, was no longer fit either for respiration or for combustion;
animals being introduced into it were suffocated in a few seconds, and when a taper was plunged into it, it was extinguished
as if it had been immersed in water.
In the next place I took 45 grains of red matter formed during
this experiment, which I put into a small glass retort, having a proper apparatus for receiving such liquid, or gaseous product,
as might be extracted. Having applied a fire to the retort in the furnace, I observed that, in proportion as the red matter
became heated, the intensity of its colour augmented. When the retort was almost red hot, the red matter began gradually to
decrease in bulk, and in a few minutes after it disappeared altogether; at the same time 41.5 grains of running mercury were
collected in the recipient, and 7 or 8 cubical inches of elastic fluid, greatly more capable of supporting both respiration
and combustion than atmospherical air, were collected in the bell-glass.
A part of this air being put into a glass tube about an inch
dilater, showed the following properties: A taper burned in it with a dazzling splendour, and charcoal, instead of consuming
quietly as it does in common air, burnt with a flame, attended with a decrepitating noise, like phosphorus, and threw out
such a brilliant light that the eyes could hardly endure it. This species of air was discovered almost at the same time by
Dr. Priestley, Mr. Sheele, and myself. Dr. Priestley gave it the name of dephlogisticated air, Mr. Sheele called it
empyreal air; at first I named it highly respirable air, to which has since been substituted the term of vital
air. We shall presently see what we ought to think of these denominations.
In reflecting upon the circumstances of this experiment, we
readily perceive, that the mercury, during its calcination, absorbs the salubrious and respirable part of the air, OK, to
speak more strictly, the base of this respirable part; that the remaining air is a species of mephitis, incapable of supporting
combustion or respiration; and consequently that atmospheric air is composed of two elastic fluids of different and opposite
qualities. As a proof of this important truth, if we recombine these two elastic fluids, which we have separately obtained
in the above experiment, viz. the 42 cubical inches of mephitis, with the 8 cubical inches of respirable air, we reproduce
an air precisely similar to that of the atmosphere, and possessing nearly the same power of supporting combustion and respiration,
and of contributing to the calcination of metals."
".. we shall have three things to distinguish in every physical
science; the series of facts that constitute the science; the ideas that call the facts to mind; and the words that express
them. The word should give birth to the idea; the idea should depict the fact; they are three impressions of one and the same
seal; and as it is the words that preserve and transmit the ideas, it follows that the science can never be brought to perfection,
if the language be not first perfected, and that however true the facts may be and however correct the ideas to which they
give rise, they will transmit only false impressions, if there are no exact impressions to convey them. The perfecting of
the nomenclature of chemistry, considered from this point of view, consists in conveying the ideas and facts in their strict
verity, without suppressing anything that they present, and above all without adding anything to them; it should be nothing
less than a faithful mirror; for we can never too often repeat that it is not Nature, nor the facts that Nature presents,
but our own reasoning that deceives us."
In 1789 he published one of the most influential textbooks in
the history of chemistry, Traité Élémentaire de la Chimie. This explained clearly the basis of his new system of chemistry,
including its nomenclature. It was rapidly translated into English by Robert Kerr (The Elements of Chemistry, Edinburgh
1790) and into many other languages.

|
| Figure 2. The title page of "The Elements of Chemistry", 1790. |
This book has been classed in importance with Newton's Principia
(which laid the foundations of modern physics) and with Darwin's The Origin of Species (which did the same for biology).
In it Lavoisier took the ideas of Boyle on simple substances and gave his own pragmatic definition of an element, and gave
the first list of elements (see below). This was an important step forward into recognising that all matter was made up of
basic building blocks in the simple substances or elements, and this idea was to be soon consolidated by John Dalton's atomic
theory.
|
|
| Figure 3. The list of elements from Lavoisier's book. |
I cannot do justice in a short article to all Lavoisier's contributions
to chemistry, never mind to other subjects that he turned his mind to. He also laid the foundations of thermochemistry (and
thus physical chemistry), organic chemistry (with his work on combustion analysis), biochemistry and physiology (with his
work on respiration and fermentation), and agricultural science. He collaborated with many individuals and his laboratory
in the Royal Arsenal was a training ground for many chemists. He worked, amongst others, with P. Laplace, A.Seguin, de Morveau
and E. du Pont, who emigrated from France with his family during the Terror and founded the great US chemical company Du Pont
in 1802 in Delaware. Not the least of his co-workers was his wife Marie, who worked with him throughout his scientific career.
Lavoisier's wife Marie, who was 14 years younger than him, was an essential part of his scientific endeavours. Nearly all
the illustrations of Lavoisier at work show her working with him in the laboratory. She equipped herself by learning English
in order to help Lavoisier in his scientific researches, as well as managing his household. She was an attractive and accomplished
hostess, and she also translated important scientific works from English to French, acted as his research assistant in making
notes of his experiments, and drew and engraved the thirteen plates for the Traité Élémentaire de Chimie, as well as
the drawings of the laboratory showing the experiments on respiration that he was pursuing at the time of his arrest.
Lavoisier's political interests
Lavoisier's energy and ability were not confined to chemistry.
He made important contributions to economics, agriculture, gunpowder manufacture, education and public administration. Almost
as soon as he was elected to the Académie des Sciences in 1768 he was appointed secretary and given the job of writing (and
in many cases researching) hundreds of official reports on diverse topics. After that whenever a job needed doing, Lavoisier
was invited to join the relevant body, appointed secretary and became the originator and author of their reports. He seems
to have a zeal for reform and nothing he touched was left unimproved. When he appointed to the Gunpowder Commission in 1775
the production of gunpowder in France (in both quality and quantity) were in a sorry state, and France was having to import
gunpowder to carry on her wars. He totally reformed the production of gunpowder, improved its quality.
He almost certainly made it possible for France to conduct successfully
her revolutionary wars of the late 18th/early 19th century.
In 1778 he purchased a country estate at Frchines, near Blois
and there he started to apply science to agriculture for the first time. He set up the first experimental farm and over a
period of ten years systematically improved the land and measured every bit of produce.
He also proposed schemes to reform public education, taxation,
local government, old age insurance, and at his death was involved in the Commission for Weights and Measures, which was to
devise the metric system.
The last act
After making all efforts to avoid being arrested by the Revolutionary
Tribunal for his membership of the Farmers-General, Lavoisier was finally arrested, together with his father-in-law, and put
in prison on the 28th. November 1793. He and his fellow farmers-general had been arrested on allegations of financial misconduct,
for which there was no evidence, and on the spurious charge of selling tobacco with too much water. But anyone with a connection
with the old regime, who had held government office, or who was wealthy or had aristocratic connections or had made enemies,
was in danger. Lavoisier never gave up hope of release until the last, and counselled his fellow prisoners against suicide.
He wrote letters explaining his case, and some of his former colleagues made representations on his behalf - but there was
a glaring silence from several of his fellow scientists who concurred in his death. McKie judges that "Lavoisier's fellow-scientists
in the Convention were morally responsible for his death".
At the end the formalities were rushed through and Lavoisier
barely had time to write one last letter to his cousin, quoted below.
"I have had a fairly long life, above all a very happy one,
and I think that I shall be remembered with some regrets and perhaps leave some reputation behind me. What more could I ask?
The events in which I am involved will probably save me from the troubles of old age. I shall die in full possession of my
faculties, and that is another advantage that I would count among those I have enjoyed. ....
So it is true that the practice of every social virtue, important
services to one's country, a life spent advantageously in the advancement of the useful arts and of human knowledge, are not
enough to protect a man from a sinister end or to avoid dying like a criminal!"
Letter by Lavoisier from prison to his cousin Augez de Villers,
7/5/1794
After a perfunctory trial on trumped charges, Lavoisier and 27
fellow farmers-general, including his father-in-law Paulze, were executed by guillotining in the Place de la Révolution on
May 8th. 1794. They were innocent victims, amongst many others, of the reign of terror that swept France in those days. Lavoisier
in his hour of need became the victim of men he had offended by his outspoken honesty, men like Marat and Foucroy (a fellow-chemist),
and his scientific colleagues did not speak up for him. Lavoisier's fame as a chemist could not save him. He was compromised
by his past, by his wealth, by his foreign and aristocratic connections, by his financial dealings, and by the enemies he
had made, and in the end all his achievements for France counted for nothing. The bodies of the victims were thrown into unmarked
graves. The confused circumstances of the time had enabled small-minded men to commit murder and to bring down a great man,
much as a pack of mongrels might bring down a noble stag caught in a forest.
"Thus died France's greatest scientist whose labours brought
chemistry a new and logical theory, a new and systematic nomenclature, a fresh and scientific outlook, and who had swept away
all vestiges of alchemy and superstition. In addition to this remarkable achievement his pioneering work in agriculture, economics,
finance, politics, sociology, education, and hygiene all bore the indelible stamp of his impeccable logic, keen practical
sense, and strong appreciation of the urgent needs for constructive changes in these fields."
Denis I. Duveen
in Great Chemists Interscience, New York and London 1961
The act of a moment was soon regretted when the blood-lust of
the revolution cooled. Only two years later we find Fourcroy, who seems to have had a hand in sending Lavoisier to his death,
changing sides and giving a funeral oration on October 22nd. 1795. Soon after his arrest Lavoisier's property was seized and
inventoried after his death and was in the process of being disposed of. Madame Lavoisier and the other victim's widows appealed
and managed to obtain restitution of the seized property, including nearly all Lavoisier's papers, books and equipment in
the summer of 1795. This means that we still have all Lavoisier's original notebooks, papers, books etc., which could so easily
have been lost as his body was.
Madame Lavoisier prepared his unfinished Memoirs for publication,
the first two volumes of which were already in proof when he died. They were published privately in 1805. On October 22nd
1805 Madame Lavoisier married another scientist, the American physicist Benjamin Thompson, also known as Count Rumford. She
insisted on being known as the Countess Lavoisier-Rumford. The marriage was not happy and broke up after four years. Both
before and after her second marriage she continued to hold salons for the leaders of science in Europe, but she did not invite
those French scientists who failed to use their influence the save her husband. She died on February 10th. 1836, leaving no
descendants.
Lavoisier's achievements
"Lavoisier changed the whole structure and outlook of chemistry,
the science which more than any other touches our daily lives, and which affects them in almost every phase."
D. McKie p.2
Lavoisier's work laid the foundations of modern chemistry in
a number of ways:
1) His careful experimental work on combustion and oxidation
provided a better theory which fitted more facts, and replaced the old phlogiston theory, which had been a fruitful hypothesis
but had outlived its day.
"..I am at the point of attacking the entire doctrine of Stahl
concerning phlogiston, and of undertaking to prove that it is erroneous in every respect.."
(Lavoisier, 1777)
2) He established the importance of quantitative experimental
work as a basis for chemical theory.
"We must trust in nothing but facts. These are presented to
us by nature and cannot deceive. We ought in every instance to submit our reasoning to the test of experiment. It is in those
things which we neither see nor feel that it is especially necessary to guard against the extravagances of imagination which
forever incline us to step beyond the bounds of truth."
(Lavoisier)
3) He reformed and laid the basis of modern chemical nomenclature.
"It is now time to rid chemistry of every kind of impediment
that delays its advance; to introduce into it a true spirit of analysis; and we have sufficiently demonstrated that it is
by the perfecting of its language that this reform must be brought to pass. We are doubtless very far from knowing the whole
and all the parts of this science: it is therefore to be expected that a new nomenclature, although formed with all possible
care, must be far from perfect; but provided it has been undertaken on sound principles, provided that it is a method naming
rather than a nomenclature, it will naturally adapt itself to future discoveries; it will indicate beforehand the place and
name of new substances that may be discovered, and it will need no more than particular amendment in some details."
(Lavoisier 1787)
4) He firmly established the idea of a chemical element in his
important textbook The elements of Chemistry (English translation 1790).
"We shall content ourselves here with regarding as simple
all the substances that we cannot decompose, all that we obtain in the last resort by chemical analysis."
(Lavoisier The Elements of Chemistry 1790)
5) He established the basic processes involved in providing energy
in animals and initiated the study of the chemistry of life (later biochemistry).
"One would say that the analogy between respiration and combustion
had not escaped the poets, or rather the philosophers of antiquity whose interpreters they were. The fire stolen from heaven,
the fire of Promethium, is not merely an ingenious poetical idea; it is a faithful picture of the operations of Nature, at
least for animals that breathe: we can therefore say with the ancients that the flame of life is lit at the instant the child
draws its first breath and that it is extinguished only at death. When we consider these remarkable anticipations, we are
sometimes tempted to think that the ancients actually penetrated further than we suppose into the sanctuary of knowledge and
that the fable is, indeed, only an allegory under which they hid the great truths of medicine and physics."
(Lavoisier, Memoir 1789)
6) He established on a firm quantitative footing the law of conservation
of mass in chemical reactions.
"Nothing is created in the operations either of art or of
Nature, and it can be taken as an axiom that in every operation as equal quantity of matter exists both before and after the
operation."
(Lavoisier The Elements of Chemistry 1790)
7) He laid the foundations of thermochemistry (with Laplace).
"Al the variations of heat, either real or apparent, which
a system of bodies undergoes in changing its state, are reproduced in an inverse order when the system returns to its former
state."
(Lavoisier and Laplace 1784)
8) He laid the foundations of organic chemistry by recognising
the chemical nature of plant and animal substances, by identifying organic radicals, and by developing combustion analysis
for organic compounds.
The first full biography of Lavoisier was published in France
by E. Grimaux in 1888, which stressed his personal affairs, and M. Bertholet published another scientific biography in 1890.
Compared to Faraday, for example, Lavoisier has not been written about so extensively at a popular level. The article in the
Dictionary of Scientific Biography gives a full bibliography up to 1970. The chapter in Partington's History of Chemistry
vol. III is available in most libraries.
Even in the dark days of the German occupation, reminiscent in
some ways of the dark days of terror 150 years before, the French remembered the 200th. birthday of one of her greatest sons.
An exhibition was mounted in the summer of 1943, with a display of his apparatus, papers and other personal memorabilia.
I leave the last words to Lavoisier himself as he reflected on
the contribution of the scientist to society:
"It is not absolutely necessary, in order to deserve well
of mankind and to do one's duty to one's country, to be called to glittering public office for the organization and regeneration
of empires. The man of science in the silence of his laboratory and his study can also serve his country: by his work he is
enabled to hope that he may diminish the sum of the evils that afflict the human race, and increase enjoyment and happiness;
and were he to contribute, by the charting of new ways, only to the prolongation by some years, even by some days, of the
average life of men, he could aspire also to the glorious title of benefactor of humanity."
Lavoisier, Memoir 1789
Bibliography
It is still possible to get a reprint of the 1790 edition of
The Elements of Chemistry from Dover Publications. Frederic Holmes' detailed book on Lavoisier's work on the Chemistry
of life is still available as a remaindered item. Every history of chemistry devotes considerable space to Lavoisier and there
are substantial articles/chapters in standard references such as Partington's History of Chemistry and The Dictionary
of Scientific Biography. Some other sources consulted in writing this article are given below.
Antoine Lavoisier: Scientist, Economist, Social reformer Douglas McKie Constable,London 1952
"Antoine Laurent Lavoisier" D.I.Duveen 263-282 in Great Chemists
Interscience, New York and London 1961
"Lavoisier" ch. V in Crucibles: The story of chemistry
Bernard Jaffe Fawcett, New York 1957
"Antoine Laurent Lavoisier" ch.47 in Makers of Chemistry
E.J.Holmyard O.U.P, Oxford 1931
Lavoisier and the Chemistry of Life F.L. Holmes University of Wisconsin Press 1985
"Lavoisier" ch.7 in Chemistry to the time of Dalton E.J.
Holmyard, OUP, Oxford 1925
"Lavoisier, Antoine-Laurent" H. Guerlac in vol. VIII Dictionary
of Scientific Biography Scribners, New York 1970
"Lavoisier" ch. IX in A History of Chemistry Vol. III
J.R. Partington Macmillan, London 1962
The Elements of Chemistry A-L. Lavoisier translated by R. Kerr Dover, New York 1965
Chs. VI and VII in Essays in Historical Chemistry T.E.
Thorpe Macmillan, London 1902

|
| Antoine and Marie Lavoisier in their laboratory. |
|



